Photo Dynamic Therapy (PDT), largely employed as a clinical treatment for several malignant, premalignant, and ophthalmologic pathologies, has gained importance also like a promising antimicrobial approach. Antimicrobial PDT (aPDT) relies on the application of a photosensitizer able to absorb appropriate wavelengths in the light visible range and to react with oxygen molecules in and around cells, resulting in the production of singlet oxygen or other cytotoxic reactive oxygen species (ROS), which lead to cell death, after inducing photodamage. aPDT can efficiently kill a wide range of bacteria (both antibiotic-susceptible and multi-resistant strains), viruses, fungal and protozoan parasites without causing development of resistance. This approach is particularly advantageous against bacteria naturally producing and accumulating endogenous photosensitizers such as porphyrins and flavins, pigments involved in several essential biological functions.
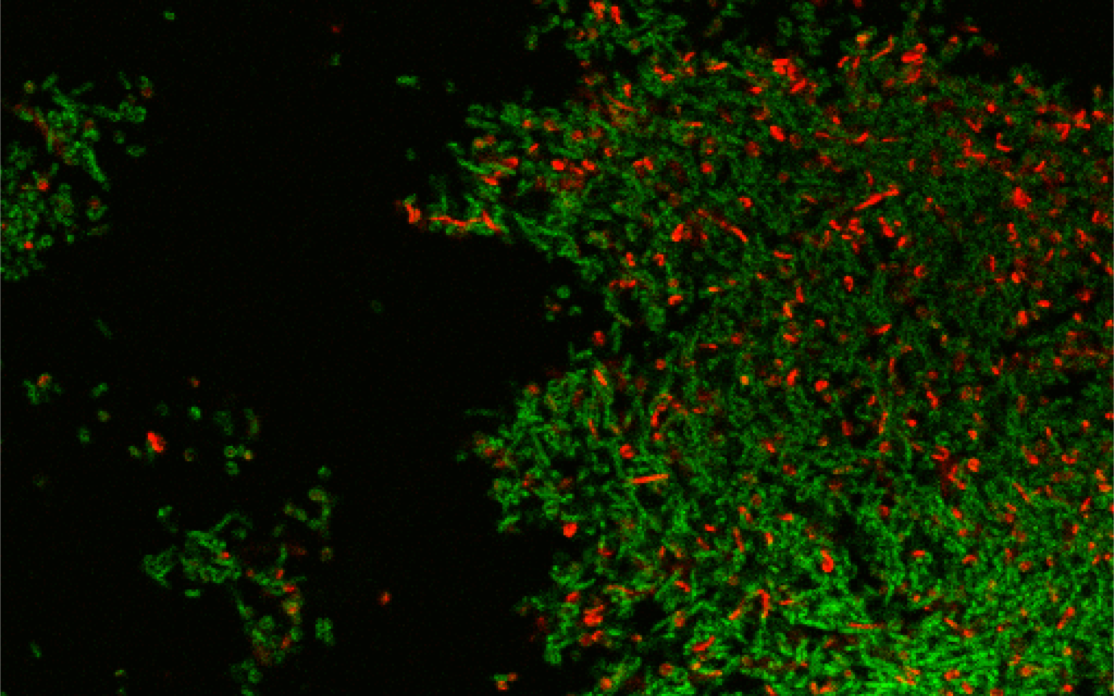
It has long been known that the vast majority of bacterial pathogens spontaneously produce fluorescing porphyrins, making them a suitable target for aPDT. In order to maximize the bacterial photokilling during the light therapy, the best illumination parameters able to activate native photosensitizers involved in the bacterial photoinactivation are determined. The phasor analysis coupled to FLIM is applied to bacterial biofilms in order to map the distribution of the bacterial porphyrins in different microenvironments with the aim to assess the extent of bacterial contamination and the presence of a biofilm inside an infected tissue, suggestive of an advanced stage of infection, thus simplifying the choice of the best treatment. This method can be also used to provide a real-time assessment and a rapid visualization of the photosensitizer distribution along with its degree of molecular packing in all the situations where it is important to know the uptake, localization pattern and interactions of the exogenously added dye to optimize the light therapy.
| People | Antonella Battisti, Giorgia Brancolini, Riccardo Nifosì, Antonella Sgarbossa*, Valentina Tozzini |
| Keywords | porphyrins; flavins; photosensitizer; antimicrobial photodynamic therapy (aPDT); light therapy; fluorescence lifetime imaging miscroscopy (FLIM); spectroscopy |
| Methods, techniques | Microbiology laboratory BSL2 plus; Biochemistry, Chemistry and Spectroscopy techniques; Confocal and TIRF microscopies. Facilities of supercomputing, Linux workstations. Software: Gaussian09, Gromacs2020, Schrodinger suites. |
| Collaborations | Policlinico San Martino di Genova, U.O Microbiologia; UniFi, Dipartimento di Scienze Biomediche, Sperimentali e Cliniche Mario Serio; The BioRobotics Institute, Scuola Superiore Sant’Anna |
| Granted projects | “CAPSULIGHT : Design of an ingestible robotic pill based on LED sources for the treatment of gastrointestinal disorders” funded by Regione Toscana – BANDO FAS SALUTE 2014, CUP B52I14005760002. |
| Publications | |
| A Battisti, P Morici, A Sgarbossa, Fluorescence Lifetime Imaging Microscopy of Porphyrins in Helicobacter pylori Biofilms Pharmaceutics 2021 | |
| P Morici., A Battisti., G Tortora, A Menciassi, G Checcucci, F Ghetti, A Sgarbossa, The in vitro Photoinactivation of Helicobacter pylori by a Novel LED-Based Device Front microbiol 2020 | |
| A Battisti, P Morici, G Tortora, A Menciassi, G Checcucci, F Ghetti, A Sgarbossa Temperature increase inside LED-based illuminators for in vitro aPDT photodamage studies Res Phys 2018 | |
| A Battisti, P Morici, F Ghetti, A Sgarbossa, Spectroscopic characterization and fluorescence imaging of Helicobacter pylori endogenous porphyrins. Biophys chem 2017 | |
| A Battisti, P Morici, G Signore, F Ghetti, A Sgarbossa, Compositional analysis of endogenous porphyrins from Helicobacter pylori Biophys chem 2017 |
*Contact person: antonella.sgarbossa@nano.cnr.it