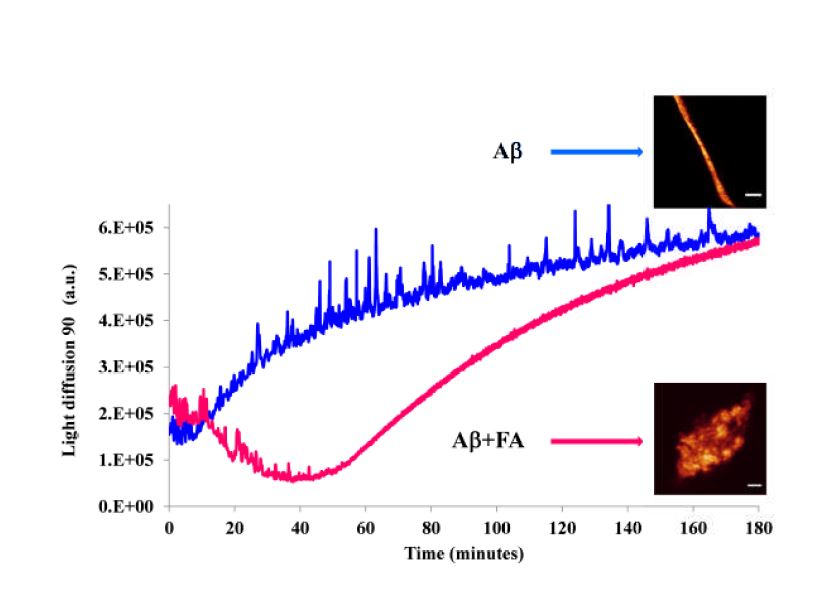
Different neurodegenerative disorders, like Huntington’s, Alzheimer’s and spongiform encephalopathy diseases, have in common the presence of insoluble protein aggregates, generally termed “amyloid,” that share several physicochemical features: a fibrillar morphology, a predominantly beta-sheet secondary structure, insolubility in common solvents and detergents, and protease resistance. Conformational constrains, hydrophobic and stacking interactions can play a key role in the fibrillogenesis process and protein–protein and peptide–peptide interactions—resulting in self-assembly phenomena of peptides yielding fibrils—that can be modulated and influenced by natural biomolecules. Small organic molecules, which possess both hydrophilic and hydrophobic moieties able to bind to peptide/protein molecules through hydrogen bonds and hydrophobic and aromatic interactions, are potential candidates against amyloidogenesis. This research activity is focused on the study of the molecular basis of the aggregation processes of neurotoxic amyloidogenic peptides responsible for Alzheimer Disease (AD) and other neurological pathologies. The inhibitory effect of natural molecules and derivatives on beta amyloid self assembly as well as the destabilizing activity on the fibril structures found in the amyloid plaques are studied by means of spectroscopic and advanced microscopy techniques. A central problem in the treatment of brain disorders is to deliver a suitable drug amount into the brain, due to the blood brain barrier (BBB) against the entry of a variety of molecules into the CNS tissue. Nanotechnology has shown promising applications in targeted drug delivery for AD, and several nanocarrier systems are under investigation.
| People | Antonella Battisti, Melissa Santi, Antonella Sgarbossa* |
| Keywords | natural molecules, beta amyloid peptide, self assembly, neurodegenerative disorders, Alzheimer disease |
| Methods, techniques | Solid phase synthesis, Rayleigh scattering, Steady state and time resolved fluorescence spectroscopy, Advanced microscopy techniques |
| Collaborations | Elisa Passaglia, CNR-ICCOM, Pisa; Maria Grazia Ortore, Dipartimento di Scienze della Vita e dell’Ambiente, Università Politecnica delle Marche ,Ancona; Silvia Vilasi, CNR-IBF, Palermo |
| Publications | |
| A Battisti, A Palumbo Piccionello, A Sgarbossa, S Vilasi, C Ricci, F Ghetti, F Spinozzi, A Marino Gammazza, V Giacalone, A Martorana, A Lauria, C Ferrero, D Bulone, MR Mangione, P L San Biagio, MG Ortore Curcumin-like compounds designed to modify amyloid beta peptide aggregation patterns RCS adv 2017 | |
| A Sgarbossa, D Giacomazza M Di Carlo Ferulic acid: A hope for Alzheimer’s disease therapy from plants Nutrients 2015 | |
| E Bramanti, L Fulgentini, R Bizzarri, F Lenci, A Sgarbossa β-amyloid amorphous aggregates induced by the small natural molecule ferulic acid J Phys Chem B 2013 | |
| A Sgarbossa, S Monti, F Lenci, E Bramanti, R Bizzarri, V Barone The effects of ferulic acid on β-amyloid fibrillar structures investigated through experimental and computational techniques Biochim Biophys Acta 2013 | |
| A Sgarbossa Natural biomolecules and protein aggregation: emerging strategies against amyloidogenesis Int J Mol Sci 2012 |
*Contact person: antonella.sgarbossa@nano.cnr.it